

Dithionite-reduced methyl viologen supported the highest rates of CO2 and COS reduction, and the stimulation of CO2 reduction (170-fold increased rate over dithionite alone) was much more dramatic than the stimulation of COS reduction (2.6-fold increased rate over dithionite alone). Although, as we shall see in Chapter 5, atoms other than carbon can have profound effects on the reactivity of.
The peak positions for all N-CNTs in Fig. nitrogen is very close to the accepted value (284.3 eV) for pure sp 2 CC bonding in pristine highly oriented pyrolytic graphite (HOPG) 40, indicating carbon atoms are almost exclusively sp 2 hybridized in non-doped CNTs. A variety of low-potential reductants, including dithionite, titanium(III) citrate, and dithionite-reduced viologens (methyl and benzyl), were suitable electron donors for the reduction of CO2 and COS. In any organic reaction, carbon plays a part. The peak position at 284.4 eV for CNTs with 0.0 at. The addition of hemoglobin to the assays also allowed the formation of CO to be monitored in real time by following the decrease in absorbance at 433 nm resulting from carboxyhemoglobin formation.
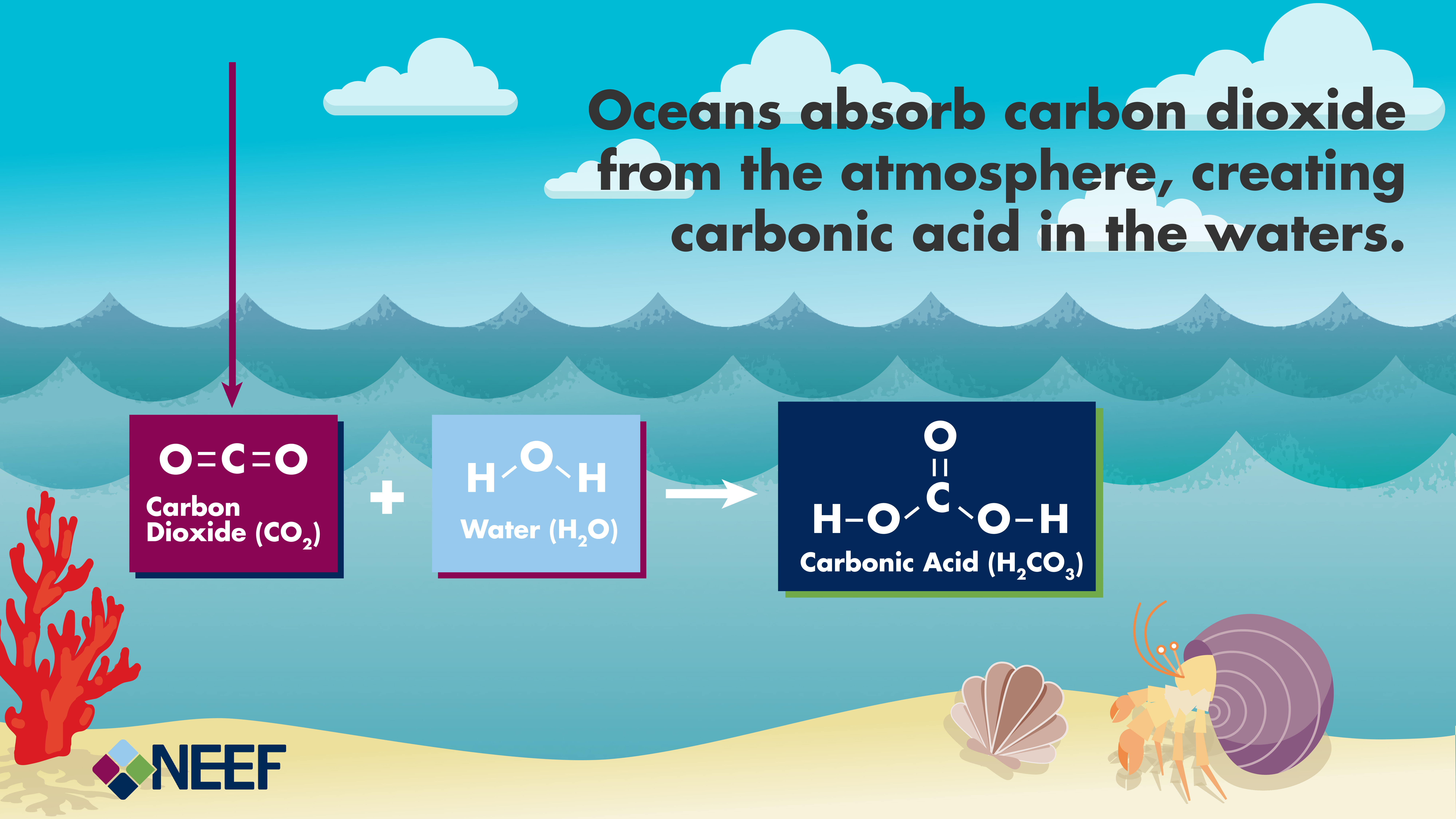
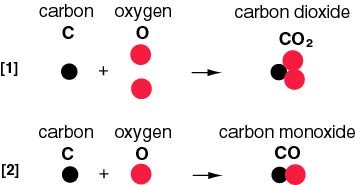
CO was a potent inhibitor of CO2 reduction at dissolved concentrations as low as 1 microM, but this inhibition could be prevented by quantitatively trapping CO as it was formed by including reduced hemoglobin in the assays. CO2 was reduced to CO and H2O, while COS was reduced to CO and H2S.
#Carbon reactivity free#
Both CO2 and COS were substrates for CODH in a reductant-dependent reaction resulting in the formation of CO. The only reaction for alkanes I can think of off the top of my head is free radical halogenation, but both hexane and cyclohexane have secondary carbons, so reactivity is comparable. isotope relationships can provide new insight into the compositional controls on OC source and residence time.The reactivities of CO2 and the related compounds COS and CS2 with the nickel- and iron- sulfur-containing carbon monoxide dehydrogenase (CODH) from Rhodospirillum rubrum have been investigated. We therefore propose the E distribution as a novel proxy to describe OC thermal reactivity and suggest that E vs. Basi cally there are two major issues in controlling the reactivity of carbon: i) reduction of the gasification rate of carbon materials in hostile environment. By analyzing a decarbonated test sample at multiple masses and oven ramp rates, we show that OC decay during RPO analysis follows a superposition of parallel first-order kinetics and that resulting E distributions are independent of experimental conditions. RPO analysis directly compares thermal reactivity to isotope composition by determining the E range for OC decaying within each temperature interval over which CO₂ is collected.
#Carbon reactivity serial#
Here, we apply this method to ramped temperature pyrolysis or oxidation (RPO) analysis but note that this approach is broadly applicable to any serial oxidation technique. We present a regularized inverse method to estimate the distribution of OC activation energy (E), a proxy for bond strength, using serial oxidation. It is therefore necessary to properly assess serial oxidation kinetics before utilizing decay profiles as a measure of OC reactivity. However, observed decay profiles depend on experimental conditions and oxidation pathway. Serial oxidation coupled with stable carbon and radiocarbon analysis of sequentially evolved CO₂ is a promising method to characterize the relationship between organic carbon (OC) chemical composition, source, and residence time in the environment.


 0 kommentar(er)
0 kommentar(er)
